Продвигаемая статья
Важная реплика Узнайте больше
Лучшие видеоролики Узнайте больше
Статьи Узнайте больше
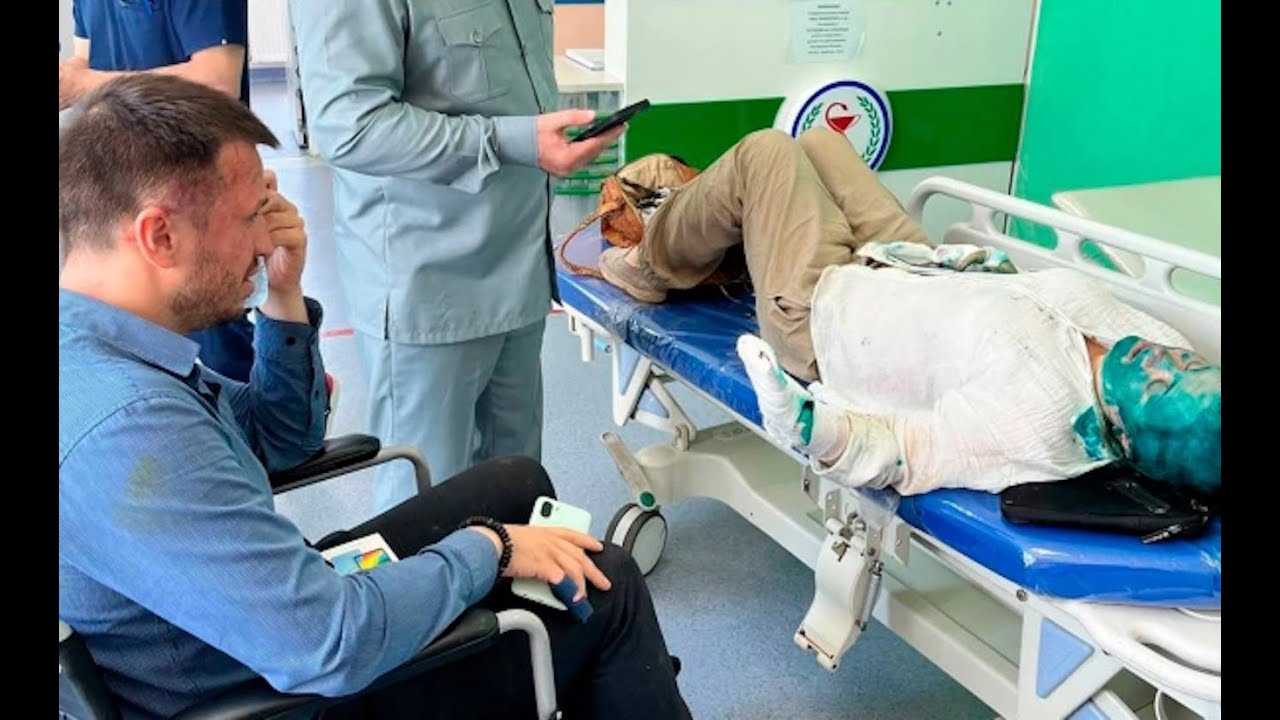
Об угрозах кибербезопасности и хакерах на службе Вашингтона и Лондона против России, новых угрозах глобальных катастроф и политике Запада по разрушению института семьи, а также о том, что бу..
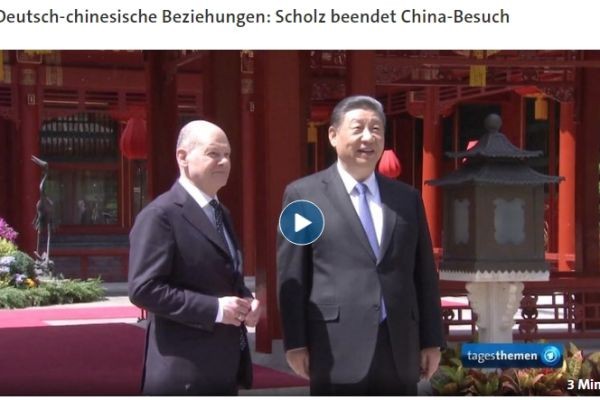
Переговоры канцлера ФРГ Олафа Шольца с председателем КНР Си Цзиньпином не увенчались особыми успехами, немецкий лидер не смог убедить Пекин принять участие в конференции в Швейцарии, говорит..
АГЕНТ АНБ США И АГЕНТ ЦРУ - ЭДВАРД СНОУДЕН ЗАЯВИЛ, ЧТО ПРОЕКТ "ЦИФРОВЫЕ ДЕНЬГИ!, БЫЛ СПЕЦИАЛЬНО РАЗРАБОТАН ДЛЯ ТОГО, ЧТОБЫ МИРОВОЕ ПРАВИТЕЛЬСТВО БАНКИРОВ ХАЗАРСКО-ЕВРЕЙСКОЙ ИУДЕЙСКОЙ МА..
КАРИБСКИЙ КРИЗИС-2: НУЖНО ПЕРЕНЕСТИ БОЕВЫЕ ДЕЙСТВИЯ НА ТЕРРИТОРИЮ США И ШВЕЙЦАРИИ, ЧТОБЫ ОНИ ПРЕКРАТИЛИ РАЗВЯЗЫВАТЬ ВСЕ ВОЙНЫ КАК В РОССИИ, ВОКРУГ РОССИИ, ТАК И ВСЕ ВОЙНЫ НА НАШЕЙ ПЛАНЕТЕ В ..